Giving patients an informed choice based on the evidence for the risks and benefits of different treatments has shown that a larger number of patients with knee osteoarthritis would prefer a PKR than the current usage rates in most countries suggest. In order to deliver more patient-centred care, the authors recommend that any decisions about arthroplasty treatment should be based on both the treatment indications and the patient’s preferences.1
“A discrete-choice experiment was developed with the following attributes: chance of complications, functional ability, awareness of the knee implant, and chance of needing another operation within 10 years.” Patients with end-stage knee osteoarthritis, aged between 40 and 80 years, completed 1 of 2 surveys depending on their self-reported functional ability (good vs fair/poor) as measured by the Oxford Knee Score.1
The completed surveys from 258 patients (92 males & 164 females) were analysed. There were 72 patients in the good-function cohort and 186 in the fair/poor-function cohort.1
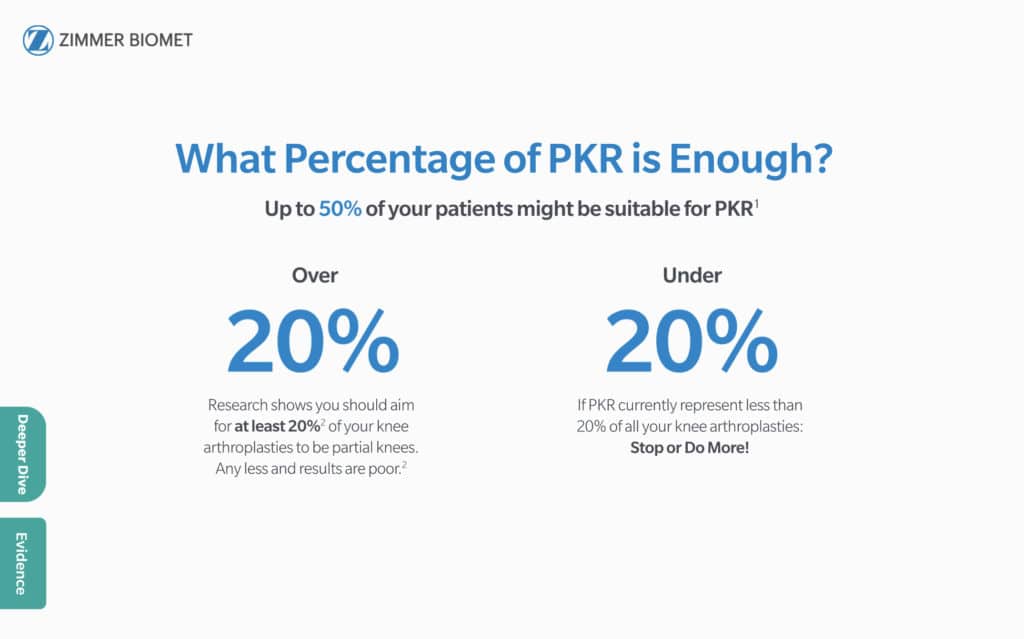
“In the good-function cohort, 42% of respondents chose TKR and 58% chose PKR. In the fair/poor-function cohort, 54% chose TKR and 46% chose PKR.” 1
This study supports the recommendation from NICE that all patients with isolated medial compartment osteoarthritis be offered a choice of PKR or TKR; because they believe that patients perceive the relative risks and benefits of treatments differently according to their personal circumstances and priorities. 2